-
 Biosafety
Biosafety
Biosafety indicates a series of actions to properly use biological knowledge and experimental technique, equipment, and facilities to protect laboratory researchers, laboratories, and surrounding environments from potential infectious organisms or biohazards.
-
 Basic element of biosafety
Basic element of biosafety
The basic elements of biosafety to prevent or minimize biohazards are proper physical containment, risk assessment capability of researchers or research organizations, and risk management operation for safety management.
-
 Proper biosafety management such as laboratory biosafety instruction, equipment, and containment by classifying microorganisms into four risk groups in terms of pathogenicity, infectivity, and risk
Proper biosafety management such as laboratory biosafety instruction, equipment, and containment by classifying microorganisms into four risk groups in terms of pathogenicity, infectivity, and risk
and setting the biosafety level of the laboratory with four levels. -
 Definition of the microorganism risk group
Definition of the microorganism risk group
Division Definition Microorganism Risk Group 1 An organism that is unlikely to cause disease in healthy adult humans. E. coli Risk Group 2 A microorganism that can cause disease but is unlikely to be a serious hazard to people. Effective treatment and preventive measures are easily available Vibrio cholerae
Enteropathogenic E. coli
Hepatitis virus
Measles virusRisk Group 3 A microorganism that usually causes disease and is likely to be a serious or fatal hazard to people. Effective treatment and preventive measures are available Bacillus anthracis
Brucella abortus
Yersinia pestis
SARS virus
Yellow fever virusRisk Group 4 A pathogen that usually causes disease and is a very serious or fatal hazard to people. Effective treatment and preventive measures are not usually available Ebola virus
Marburg virus
Lassa virus
Hendra-like virus- See Guidelines for Laboratory Biosafety of the National Institute of Health of the Korea Centers for Disease Control & Prevention
-
 In preparation for the ratification of the protocol, Korea enacted and promulgated the “TRANSBOUNDARY MOVEMENT, ETC. OF LIVING MODIFIED ORGANISMS ACT (in short, LMO Act)” in March 2001.
In preparation for the ratification of the protocol, Korea enacted and promulgated the “TRANSBOUNDARY MOVEMENT, ETC. OF LIVING MODIFIED ORGANISMS ACT (in short, LMO Act)” in March 2001.
After the ratification of the protocol in October 2007, the LMO Law started to be enforced from January 1, 2008.
-
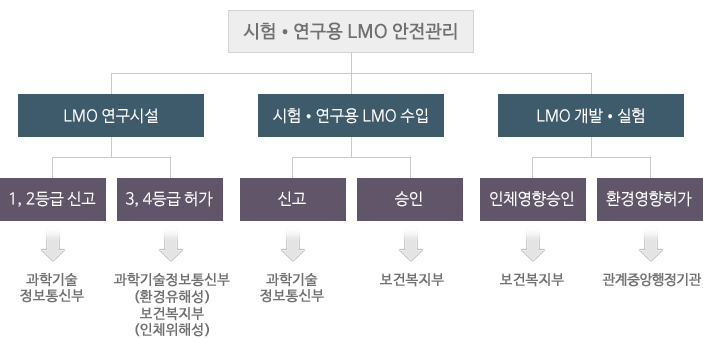
 The LMO Consolidated Public Notification contains the standard and procedure of permitting and reporting the installationㆍoperation of LMO research facilities, the standard of installing and operating the research facilities
The LMO Consolidated Public Notification contains the standard and procedure of permitting and reporting the installationㆍoperation of LMO research facilities, the standard of installing and operating the research facilities
necessary for the safety management of LMO research facilities. The research facilities that develop or use LMOs are classified into four levels in terms of safety management (Enforcement Decree of the LMO Act
[asterisk 1]) and their report or permission should be done for each level. -
 Classification of the safety management level of LMO research facilities
Classification of the safety management level of LMO research facilities
Level Target Reporting/Permission Level 1 Facilities that develop or conduct experiments using LMO that is known to not cause diseases among healthy adults and LMO that is known to not cause risk to the environment Reporting Level 2 Facilities that develop or conduct experiments using LMO that might cause disease among humans, which is easily curable, and LMO that, even if discharged into the environment, might cause minor and easily treatable risk Reporting Level 3 Facilities that develop or conduct experiments using LMO that might cause disease among humans, which might have severe symptoms but is curable, and LMO that, if discharged into the environment, might cause significant but treatable risk Permission Level 4 Facilities that develop or conduct experiments using LMO that might cause disease among humans, which has fatal symptoms and is hardly curable, and LMO that, if discharged into the environment, might cause enormous and hardly treatable risk Permission - ※ Report status (Ministry of Science and Technology Information and Communication, as of February 2014): 1st grade (1,763) and 2nd grade (1,030) report
-
 In addition to the regulations on the installationㆍoperation of LMO research facilities, the LMO Consolidated Public Notification handles the LMO safety management for testsㆍresearches in relation to LMO development,ㆍexperiment approval and the report of LMO imports for testㆍresearches.
In addition to the regulations on the installationㆍoperation of LMO research facilities, the LMO Consolidated Public Notification handles the LMO safety management for testsㆍresearches in relation to LMO development,ㆍexperiment approval and the report of LMO imports for testㆍresearches.
-